Blog: How Single-Cell Multiomics is Revolutionizing Drug Discovery
The worldwide pharmaceutical sector is dynamic and highly invested in innovation. In 2021, the industry spent an impressive 238 billion U.S. dollars on global research and development. This advanced technique explores the molecular composition of individual cells, from DNA to tiny molecules.
In light of this, single-cell multiomics appears to be a promising innovation. That same year, the multiomics market was estimated to be worth USD 2.57 Bn, demonstrating the importance and expanding influence of single-cell multiomics within the sector.
Many scientists recognize single-cell RNA sequencing, but as technology advances multiomics including genomics, epigenomics, proteomics, and metabolomics have come to the forefront. While traditional drug discovery methods serve as a foundation, they often don't achieve the necessary precision and depth.
By using various omics techniques, single-cell multiomics gives a more complete view of the cellular landscape. This is much like assembling a jigsaw puzzle and signals a new phase in the drug discovery process.
Techniques in Single-Cell Multiomics
Single-cell multiomics covers a broad area, offering many techniques that let researchers study individual cells from multiple perspectives simultaneously. Single-cell RNA sequencing is one key technique, evidenced by the rising interest in single-cell RNA seq studies. This method takes individual cells and sequences their RNA, giving deep insights into their RNA profiles.
However, multiomics goes beyond RNA. It looks at other molecules that scientists also find interesting. For example:
- Epigenomics looks at changes to DNA and how DNA is accessed
- Genomics explores the DNA of individual cells to find possible changes or errors
- Proteomics studies the proteins in the cells, and
- Metabolomics examines the small molecules inside the cells
Additionally, new technologies have pushed single-cell multiomics forward. As researchers learn more, they develop better tools and methods. One popular option is studying chromatin accessibility with single-cell ATAC-seq. This method finds parts of the genome that are open and possibly active, compared to closed or inactive elements.
When it comes to finding new drugs, these techniques transform the process. By looking closely at individual cells, they help create more specific and effective treatments.
Applications in Drug Discovery
Single-cell multiomics doesn't isn’t just for basic research; it has a significant impact on drug discovery. As we study cells more closely, multiomics gives us important information for drug research with many applications.
Target Identification
One of the primary goals of drug discovery is to identify potential targets for therapeutic intervention. Single-cell multiomics helps us pinpoint these uses. By looking at individual cells closely, we can locate specific pathways or molecules that are altered when a disease strikes. This helps develop drugs that specifically target the areas where they're needed, leading to more effective treatments.
Biomarker Discovery
Biomarkers are indicators of normal or pathological processes in the body. Through multiomics, we can discover novel biomarkers that help in disease diagnosis and prognosis, and even monitor the effectiveness of treatments. By understanding the unique molecular signature of a disease, we can develop tests that detect these biomarkers, enabling early diagnosis and timely intervention.
Personalized Medicine
Every individual is unique, and so is their genetic makeup. Single-cell multiomics provides a window into this genetic diversity, allowing for treatments tailored to individual genetic profiles. By understanding the molecular intricacies of a patient's cells, we can anticipate their response to specific drugs, allowing tailored treatment for their condition's efficacy.
Single-cell multiomics analysis provides unprecedented detail about the cellular world, paving the way for breakthroughs that could revolutionize medicine. The FDA's growing approval of customized medicines serves as further evidence of the need for personalized medicine. This trend is outlined in a report by the Personalized Medicine Coalition.
|
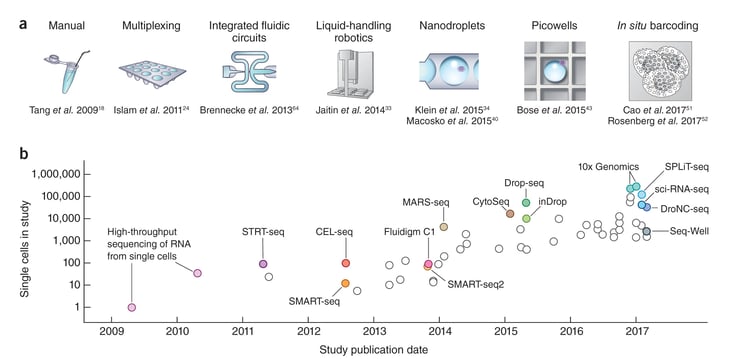
"Scaling of scRNA-seq experiments. (a) Key technologies that have allowed jumps in experimental scale. A jump to ∼100 cells was enabled by sample multiplexing, and then a jump to ∼1,000 cells was achieved by large-scale studies using integrated fluidic circuits, followed by a jump to several thousands of cells with liquid-handling robotics. Further orders-of-magnitude increases bringing the number of cells assayed into the tens of thousands were enabled by random capture technologies using nanodroplets and picowell technologies. Recent studies have used in situ barcoding to inexpensively reach the next order of magnitude of hundreds of thousands of cells. (b) Cell numbers reported in representative publications by publication date. Key technologies are indicated. |
Challenges and Responses
Single-cell multiomics holds great promise for drug discovery, but it also brings challenges. Still, the science community keeps pressing forward, finding new ways to push the boundaries of what's possible.
Harnessing Big Data with Advanced Computational Tools
The sheer volume of data generated from single-cell multiomics experiments is enormous. As we move forward, bioinformatics will continue to evolve with more sophisticated algorithms capable of processing and integrating large datasets. These tools will be designed to decipher complex biological networks and pathways, paving the way for the identification of novel drug targets.
Cloud Computing and Collaborative Frameworks
Cloud-based bioinformatics platforms are becoming increasingly crucial, allowing researchers to store, share, and analyze massive datasets effectively. Collaborative frameworks by cloud technologies will facilitate multi-institutional research, leveraging the expertise and computational resources globally to accelerate the research that fuels drug discovery.
It will be necessary to create specialized cloud-based platforms for single-cell analysis. These platforms should be easy to use yet flexible enough to adapt to the evolving algorithms and approaches used in data analysis.
Machine Learning and AI-driven predictive Models
Since single-cell data is a collection of many messy, labeled observations, and its format is a sparse matrix. These factors make it an approach particularly well-suited for machine learning (ML) and artificial intelligence (AI) analysis techniques. As the data continues to grow, these technologies will become central to interpreting single-cell multiomics data. Predictive models using AI will help in understanding disease progression and drug response for known and novel cell populations. Before they enter into clinical trials, these new models will be crucial in predicting the efficacy and toxicity of new drug candidates.
Educational and Skill Development
The demand for skilled bioinformatics, data science, and computational biology professionals is already growing, but the breadth of knowledge required to develop single-cell solutions is daunting. Next generation biologists, bioinformaticians, data scientists, and software engineers will need to learn to work in interdisciplinary teams to tackle these types of problems.
Solutions and Best Practices
We must establish best practices for single-cell multiomics research. Standardizing protocols, ensuring reproducibility, and fostering collaborations between researchers, clinicians, and bioinformaticians keep the promise of single-cell multiomics alive. As we continue exploring this field, the emphasis on innovation, collaboration, and ethical considerations will guide us.
Using advanced methods, especially with genetic data, raises ethical questions. People worry about keeping their genetic data private, permitting its use, and how others might misuse this information. Establishing strong ethical guidelines and transparent research methods is crucial. Informed consent, data anonymization, and strict data access controls are some steps taken to address these concerns.
To navigate these complexities, such frameworks proposed by the Global Alliance for Genomics Health offer guidelines for responsible sharing of genomic and health-related data.
Real-World Applications
The promise of single-cell multiomics is undeniably exciting, and real-world possibilities show its full potential. Across the globe, researchers and pharmaceutical companies are exploring multiomics to bring about tangible changes in drug discovery.
Cancer Research
For instance, by analyzing tumor cells' genetic and molecular profiles, researchers are working to develop targeted therapies for specific cancer subtypes. Tailored to the unique genetic makeup of the tumor, these drugs will offer increased efficacy and reduced side effects compared to traditional chemotherapy. An example of this application is seen in colon cancer research. In a study of Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer report published on Cell.com, single-cell RNA sequencing was utilized to dissect the cellular landscape of tumor-infiltrating myeloid cells.
Neurodegenerative Diseases
The study of neurodegenerative diseases is another notable example of this. Traditional drug discovery methods often hit a roadblock due to the complexity of the brain's cellular makeup. However, with the advent of single-cell multiomics analysis, researchers have been able to dissect the molecular profiles of individual neurons. As a result, they have been able to identify specific pathways that are changed in diseases like Parkinson's and Alzheimer's. This is paving the way for drugs that target these particular pathways, offering hope for more effective treatments in the future.
Autoimmune Diseases
In autoimmune diseases like rheumatoid arthritis or lupus, researchers have identified specific molecular signatures that differentiate the diseases. This could improve diagnostic accuracy and lead to the development of drugs targeting the unique signatures, providing more effective treatment options.
Future Perspectives
The journey of single-cell multiomics in drug discovery is just beginning, and the horizon is filled with possibilities. This upward trajectory is expected to continue, with projections showing that the global single-cell analysis market could reach USD 5.6 billion by 2025. As we look to the future, several emerging trends and technologies promise to enhance the impact of multiomics further and reshape the pharmaceutical research landscape.
Emerging Technologies
New techniques are continually developed in biotechnology that promise to refine and expand our understanding of individual cells. For instance, spatial transcriptomics, which provides a spatial context to gene expression data, is gaining traction. This technique adds another layer of complexity to our understanding by enabling researchers to determine what genes are expressed and where within a tissue.
Potential Impact
Single-cell multiomics will likely change more than just drug discovery. As we use this method more, it could change many parts of healthcare. We might see more personalized medicine, where doctors choose treatments based on a person's unique genes and molecules. Also, by finding clear signs of diseases early through multiomics, we could diagnose and treat people earlier, leading to better results and lower costs.
Moreover, as we unravel the human body's mysteries at a cellular level, our understanding of disease mechanisms will become more nuanced. This could pave the way for preventive medicine, where interventions occur even before a disease takes hold, based on the patient’s genetic and molecular risk factors.
Breakthrough Potential
Single-cell multiomics have a bright future in drug discovery and healthcare. At this juncture, the potential for breakthroughs and innovations is immense, promising more accurate, personalized, and proactive healthcare.
However, as with any pioneering technique, challenges exist. While we face significant challenges, they also present opportunities for scientific discovery. The scientific community's resilience and ingenuity will help find the solutions and expand the limits of what we can achieve.

Mark Kunitomi
Mark Kunitomi is the Chief Scientific Officer at Almaden Genomics. He was a post-doctoral fellow at UC San Francisco with a background in genomics, bioinformatics and microbiology, and he has a Ph.D. in Biochemistry & Molecular Biology from the University of California, San Francisco.