Blog: Single-Cell Epigenomics Methods, Challenges & Applications
Single-cell epigenomics has emerged as a pivotal tool in cellular biology, reshaping our comprehension of diseases, development, and gene interactions. This approach focuses on studying epigenetic modifications at an individual cell level rather than examining a multitude of cells collectively. It allows us to see the forest and the trees, capturing the individuality of each cell while still understanding its role in the larger biological tapestry. Here are some of the methods, challenges, and applications of single-cell epigenomics the science community is exploring.
Exploring Single-Cell Epigenomics
By understanding "cell-to-cell variability" and the distinct patterns within each cell, the significance of single-cell epigenomics in current research becomes clear. This paves the way for personalized medicine and more targeted therapeutic strategies. These epigenetic changes don't alter the DNA sequence but influence how genes are expressed or regulated. Insights into how genes turn "on" or "off" and how this regulation can vary amongst cells within the same organism are provided by examining the modifications. Focusing on individual cells not only adds to but refines our knowledge, ensuring that our gene regulation understanding is as precise and comprehensive as possible.

|
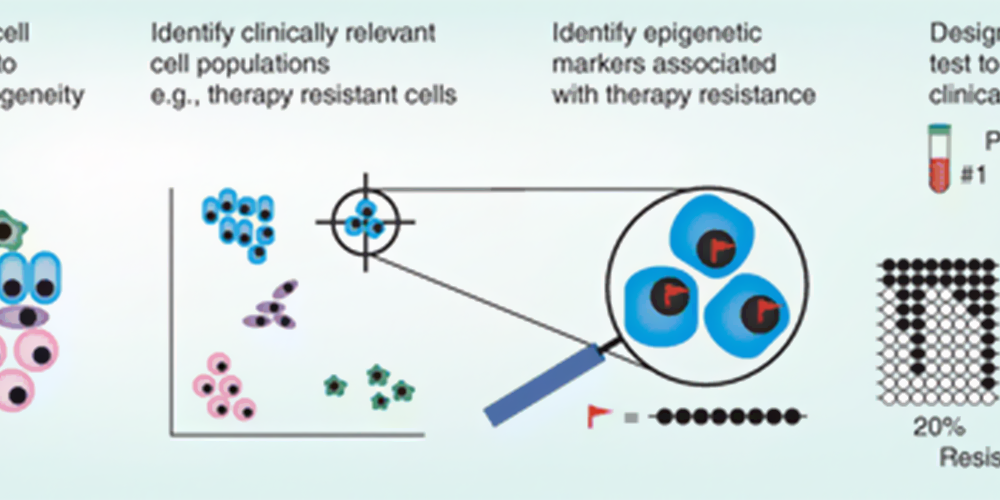
Cellular heterogeneity in cancer is associated with clinical challenges such as metastasis, therapeutic response and relapse. Single-cell technologies will identify target cell populations (by dimensional reduction methods) that could not be distinguished using traditional techniques. Single-cell epigenomics will identify modifications specific to those cells. For example, DNA methylation at a certain allele may be observed only in therapy resistant cells (red flag). Based on this information, simplified tests will be developed for clinical use. For example, amplicon bisulphite sequencing of specific loci may be used to advise clinicians of the proportion of cells in a sample with therapeutic resistance (each line indicates a sequencing read, black and white circles represent methylated and unmethylated cytosines respectively, the methylation pattern associated with therapy resistance is highlighted in red). Bond, Danielle R, et al. Figure 2. Translation of single-cell epigenomics into clinical implementation., Future Medicine, Creative Commons license,www.futuremedicine.com/doi/full/10.2217/epi-2020-0046. 29 Jan. 2024 |
Harnessing Cutting-Edge Tools and Technologies
Navigating single-cell epigenomics requires a robust set of tools and methodologies. One standout technology is ATAC-seq, or Assay for Transposase-Accessible Chromatin using sequencing. This tool provides insight into chromatin accessibility, indicating whether specific genome regions within a cell are active or dormant.
Another crucial methodology is chromatin immunoprecipitation, known as ChIP-seq. It pinpoints chromatin regions associated with specific proteins or modifications, shedding light on protein-DNA interactions and their influence on gene activity. It helps us determine whether particular genome regions within a cell are open or closed.
This "openness" or "closedness" is crucial because it indicates if a DNA segment is active or dormant, revealing gene regulation and expression in that cell. These technologies, among others, are foundational to Single-Cell Epigenomics, enhancing our understanding and paving the way for broader genomic discoveries.
Unlocking Insights through Disease Research
Single-cell epigenomics offers deep insights beyond basic research, especially in disease studies. It's pivotal in cancer research, revealing cellular differences and unique epigenetic markers influencing tumor growth and therapy resistance. This detailed view aids in developing targeted treatments and understanding tumor evolution. For instance, it's helped uncover diverse cells within brain tumors, pinpointing cells that might cause recurrence. Additionally, it provides insights into stem cell development, hinting at potential in regenerative medicine.
Addressing Current Challenges Head-On
Single-cell epigenomics offers profound insights, but it has unique challenges and considerations. Specific hurdles researchers face include:
Technical Challenges
Studying individual cells involves working with tiny amounts of genetic material, leading to potential issues like amplification biases where specific DNA fragments might be overrepresented. This can skew results. However, advancements are continually emerging, with new techniques to reduce these biases and improve quality control measures.
Sample Preparation
Researchers must undertake a complex sample preparation process to ensure cell viability, maintain DNA integrity, and accurately represent the cell's epigenomic landscape. They need to optimize every step, from cell isolation to DNA extraction. The sensitivity of this technique creates a persistent risk of contamination. However, technological advancements and new protocols continue to address these challenges. For example, the latest amplification techniques reduce biases, and teams incorporate rigorous quality control steps into their workflows.
Data Interpretation
The low signal-to-noise ratio in single-cell data makes interpretation challenging. Epigenomics sequencing and data analysis bring their level of complexity. However, developing advanced computational tools and algorithms is helping to refine the process, though it remains a complex task.
Cost and Accessibility
Lastly, cost is the elephant in the room. While single-cell epigenomics technologies break new ground, they come with a hefty price tag. Many researchers find the equipment, reagents, and software too expensive. Moreover, the limited availability of these technologies, especially in areas with restricted research infrastructure, poses a significant hurdle.
While single-cell epigenomics holds immense promise, it's essential to approach it with a clear understanding of its challenges. By acknowledging these issues and actively seeking solutions, we can tap into the full potential of this area of study.
Navigating Ethical and Regulatory Issues
Single-cell epigenomics offers more than just insights into cells and genes; it also brings forward ethical and regulatory challenges that researchers must address.
1. Addressing Data Privacy
The ability to extract detailed genetic information from a single cell raises significant ethical questions about safeguarding data privacy and measures in genomics to prevent it from falling into the wrong hands. When researchers collect human samples, they must ensure participants fully understand the study's scope and implications and provide informed consent.
2. Prioritizing Ethical Sampling
When we collect samples, especially from humans, we must prioritize ethical considerations. Researchers must ensure participants fully understand the study's goals, potential outcomes, and the use of their genetic data. Obtaining informed consent that demonstrates participants clearly understand the research is a foundational ethical practice.
3. Maintaining Regulatory Compliance
The evolving world of single-cell epigenomics comes with a changing landscape of regulations. These rules, which can vary from local to international levels, aim to uphold research integrity and protect participants. Researchers must stay current and comply with these guidelines to ensure their work meets the highest ethical and professional standards.
As we explore single-cell epigenomics, we must balance our scientific pursuits with ethical considerations and regulatory compliance, ensuring the field advances with respect and integrity.
Personalizing Medicine and Treatment
Single-cell epigenomics is transforming how we view medicine by emphasizing the unique genetic makeup of individuals. Care becomes deeply personal and tailored to the genetic story each person carries. This approach allows us to:
- Tailor Treatments: Instead of general treatments, we can design therapies based on a person's specific genetic variations. Instead of a one-size-fits-all approach, treatments can be created based on the particular epigenetic modifications in a person's cells. This means two people with the same condition might get different, optimized treatments based on their unique genetics. The implications are profound, especially for diseases such as cancer that have historically been challenging to treat due to their genetic complexity.
- Refine Disease Classifications: Beyond tailored treatments, single-cell epigenomics offers the potential for more refined disease classifications. Diseases like cancer often have multiple genetic variations. By studying these at the single-cell level, we can diagnose and classify diseases more accurately. This helps target treatments more effectively, addressing the root genetic causes rather than just the symptoms.
Single-cell epigenomics steers us towards a future where treatments address the diseases and are tailored to the individual's unique genetic story, ensuring the best outcomes.
Interacting with the Environment
Our genetic code, while a foundational blueprint, doesn't operate in isolation. It interacts constantly with the environment, responding and adapting to external cues. In single-cell epigenomics, understanding this relationship is crucial.
Everything around us impacts our epigenome, from our diet the air quality, to our stress levels. These environmental factors modify our DNA and its associated proteins without altering the DNA sequence. For example, exposure to certain toxins might add or remove methyl groups on the DNA, affecting how genes work. Likewise, our nutrition can trigger epigenetic shifts that influence our health. Individual cells might react differently to these environmental factors on a microscopic scale, creating a varied epigenetic landscape within a tissue.
While our genes provide the script, the environment often directs the performance. Recognizing and understanding the influence of environmental factors on epigenetic changes is essential for navigating single-cell epigenomics. It reminds us that our genetic story is not just written by our DNA but is co-authored by the world around us.
Welcoming a Brighter Tomorrow
The field of single-cell epigenomics offers rich potential opportunities for the future. Technological advancements are rapid. New methods promise greater precision in detecting epigenetic changes, while computational innovations help process vast data sets more efficiently. As sequencing costs drop, more researchers can access this powerful tool.
Current research projects and clinical trials in this field aim to understand diseases like Alzheimer's and the intricacies of early human development. Clinical trials use the technology to craft personalized treatments, enhancing efficacy while reducing side effects.
In medicine, single-cell epigenomics promises precise diagnoses and individualized treatments. In environmental science, it offers insights into how our surroundings shape our epigenome, guiding better health strategies. As we probe deeper, we can expect to discover unforeseen applications that solidify single-cell epigenomics as a valuable tool in science and medicine.

Mark Kunitomi
Mark Kunitomi is the Chief Scientific Officer at Almaden Genomics. He was a post-doctoral fellow at UC San Francisco with a background in genomics, bioinformatics and microbiology, and he has a Ph.D. in Biochemistry & Molecular Biology from the University of California, San Francisco.